Preparation of Alcohols
Preparation of Alcohols: Overview
This Topic covers sub-topics such as Oxymercuration Demercuration Reaction, Methods of Preparation of Alcohols, Mechanism of Acid Catalysed Hydration of Alkenes and, Preparation of Alcohols by Reduction of Aldehydes and Ketones
Important Questions on Preparation of Alcohols
Match the conversions with the reagents that are used in the process:
| i. | Phenol to benzoquinone | a. | H2O/H+ |
| ii. | Propanone to 2-methylpropan-2-ol | b. | Na2Cr2O7/H2SO4 |
| iii. | Propene to propan-2-ol | c. | CH3MgBr/H2O |
An organic compound A on reduction forms compound B . B reacts with HBr to form the compound C. C with Mg forms Grignard reagent D which reacts with A to form a product which on hydrolysis gives E. Identify A to E.
In the following sequence of reactions,
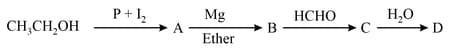
The compound D is –
Which of the following compound gives -Ethylpentan--ol by the action of ethyl magnesium iodide followed by acid hydrolysis?
Which of the following alcohols cannot be prepared by reduction of carbonyl compounds?
Which one of the following reactions would produce a secondary alcohol?
Propene on oxidation with diborane in presence of alkaline hydrogen peroxide gives _______.
Which of the following alcohol cannot be prepared by acid catalysed hydration of alkenes?
The major products and in the following reaction sequence, are
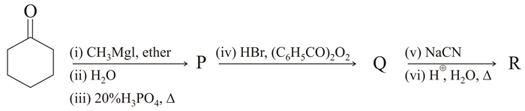
In this sequence of reaction, (Y) is :
Hydroboration oxidation, oxymercuration-demercuration and acid catalysed hydration will give same product in:-
The reduction of acetone by gives:
Correct struture of product of below reaction?
In this sequence of reactions, the starting alcohol is
For the following sequence of reactions:
The product formed will be :-
In the given reaction, what will be the final product :
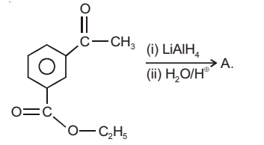
What will be the product in the above reaction?
Which of the following reagents will be suitable to obtain the product given below ?
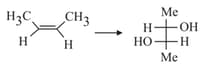
Which among the following compounds on reaction with excess methyl magnesium bromide followed by acid would not give tert-butyl alcohol?
